What is KD (dissociation constant)
Conceptually, the dissociation constant, or KD, is an equilibrium constant corresponding to the affinity between a ligand and a receptor (or between any two binding partners, such as an antibody and an antigen).
Experimentally, the dissociation constant, or KD, is the concentration of analyte (ligand) at which half of the indicator (or binding sites on the receptor) is bound.
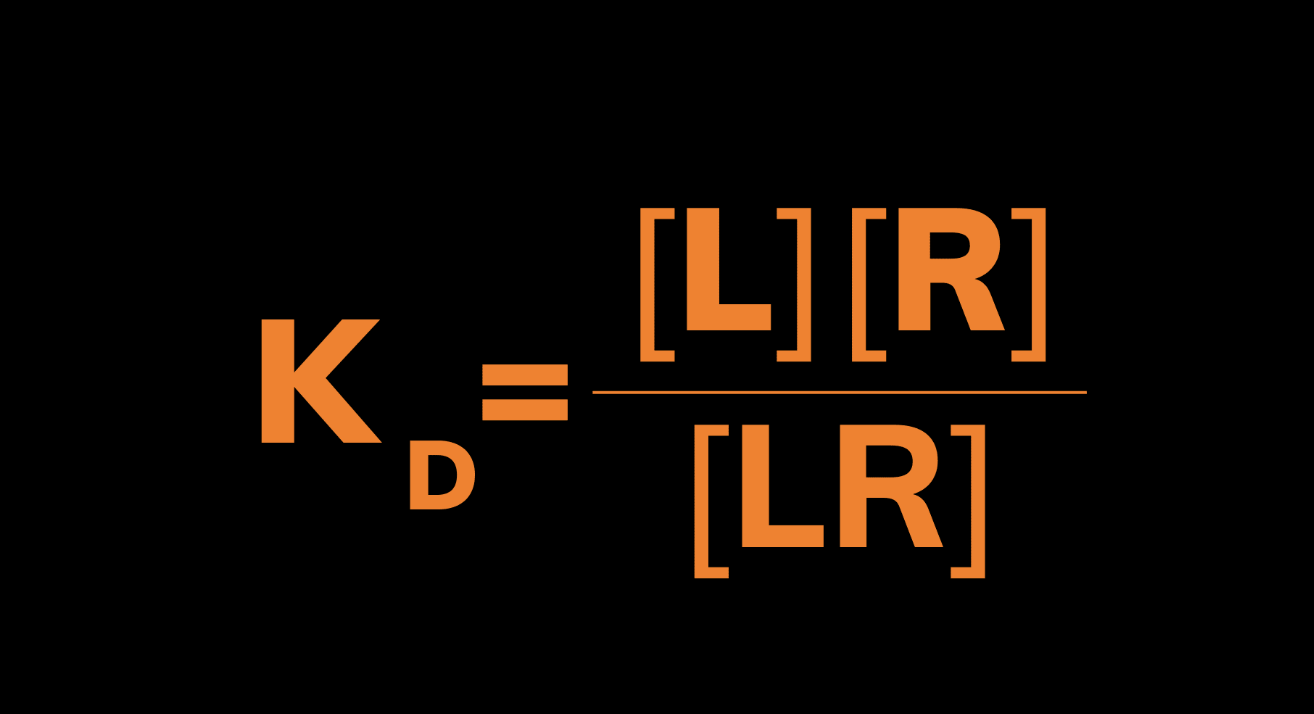
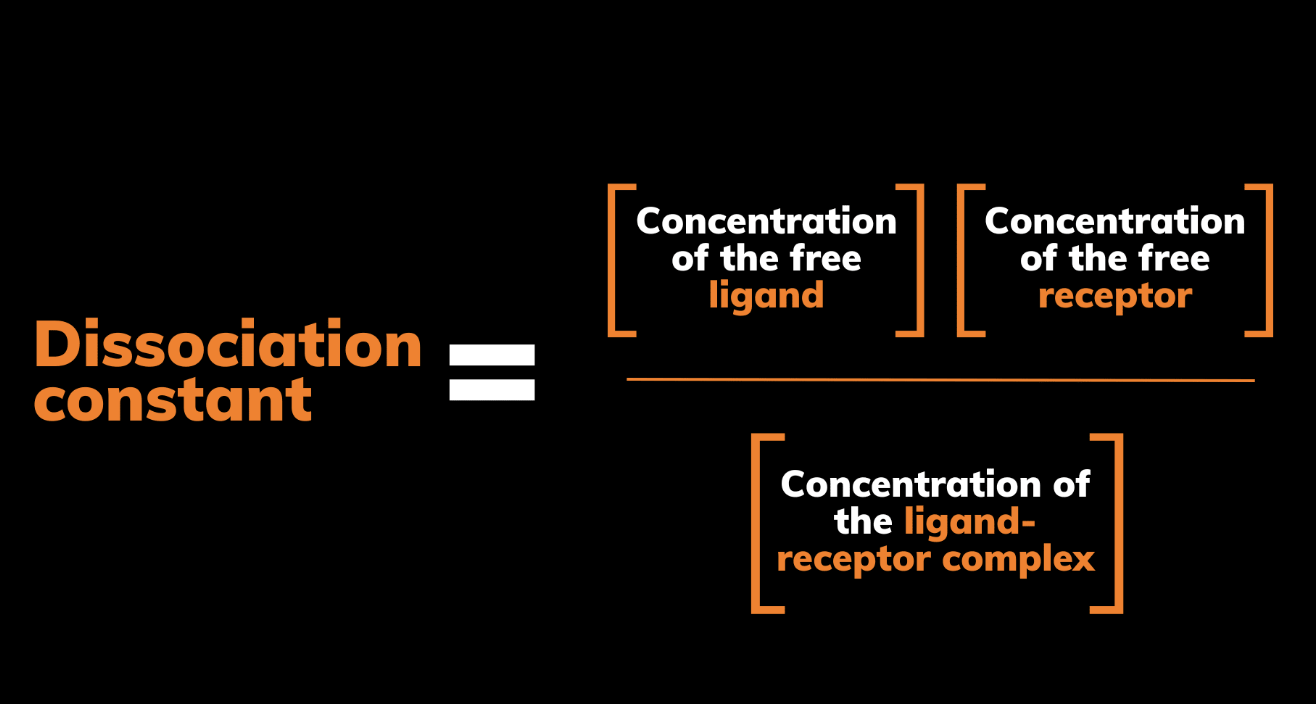
KD reflects the concentration of ligand required to occupy half of the receptor sites
Kd is a quantitative measure of binding affinity in molecular interactions, and in pharmacology affinity is often defined as a measure of the strength of drug-receptor binding. It is typically expressed in molar units (e.g., nM, μM, or M). Below you can see KD represented on a graph (in red)
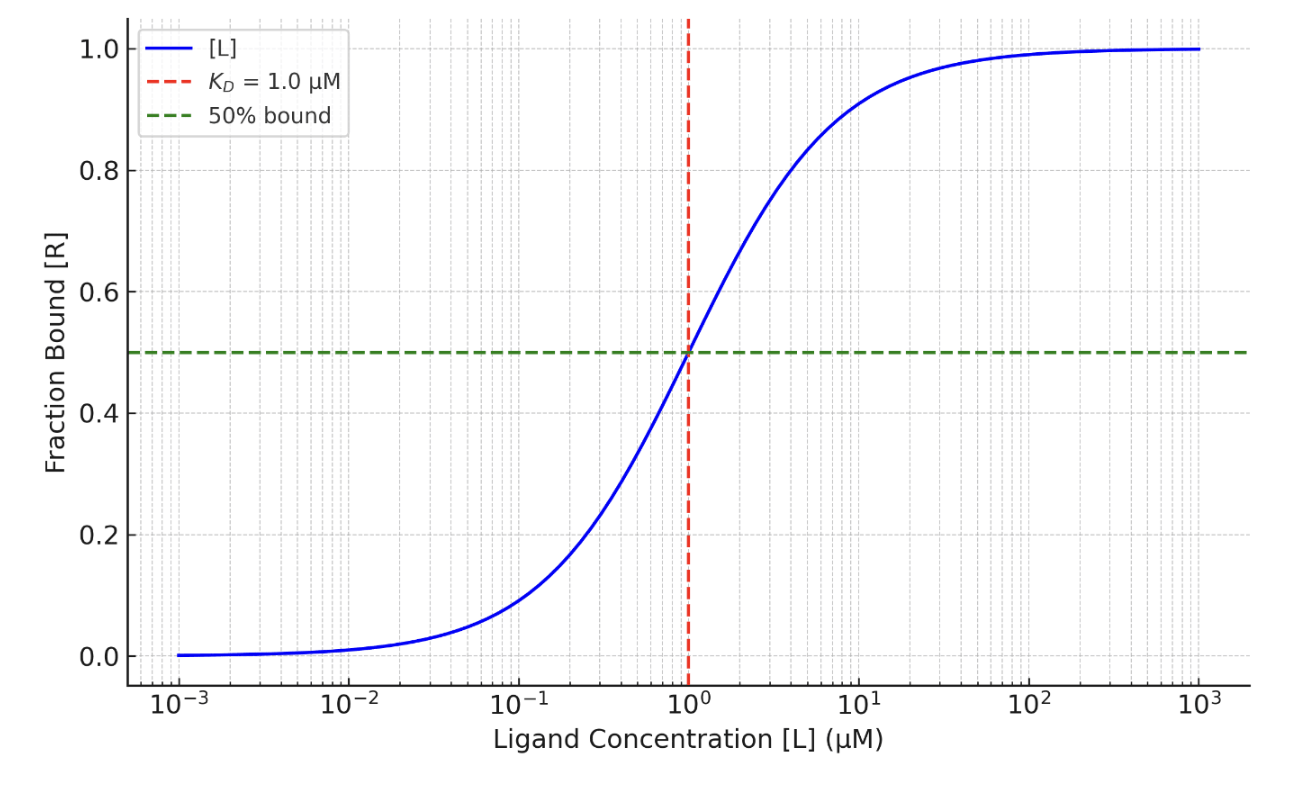
Lower KD value indicates strong binding affinity (the ligand binds more tightly to the receptor) higher KD suggests weaker binding (the ligand binds less tightly to the receptor). A small KD, such as 1 nM, means that even a tiny amount of ligand (nM) is sufficient to bind half of the available receptors. Example: Antibody-antigen interactions often have KD values in the nanomolar range. A larger KD, such as 100 μM, indicates that a higher ligand concentration is needed to achieve significant binding (10-1000 micromolar range). Example: Enzyme-substrate interactions often exhibit higher KD values compared to antibodies.