What are the different ways of setting up FIDA assays
Below you will find descriptions of the FIDA methods, remember you can always learn how to set these up and when to use which method on our e-learning platform: https://signups.motimate.app/fidabio
1. Premix:
In this methods the indicator and the analyte are mixed prior to the experiment, thus are at the equilibirum. In premix experiment there is an corresponding analyte well/vial for every indicator vial/well.
Premix sample requirements:
- Minimum 10 µL indicator per datapoint
- For each indicator a corresponding analyte with the same analyte concentration
- 50 µL analyte per data point for a triplicate
- 150 µL of analyte stock at 20 fold the expected Kd
Recommended when assaying:
- Competitive titrations (reverse titration)
- Ternary complexes
- Kinetics are slow
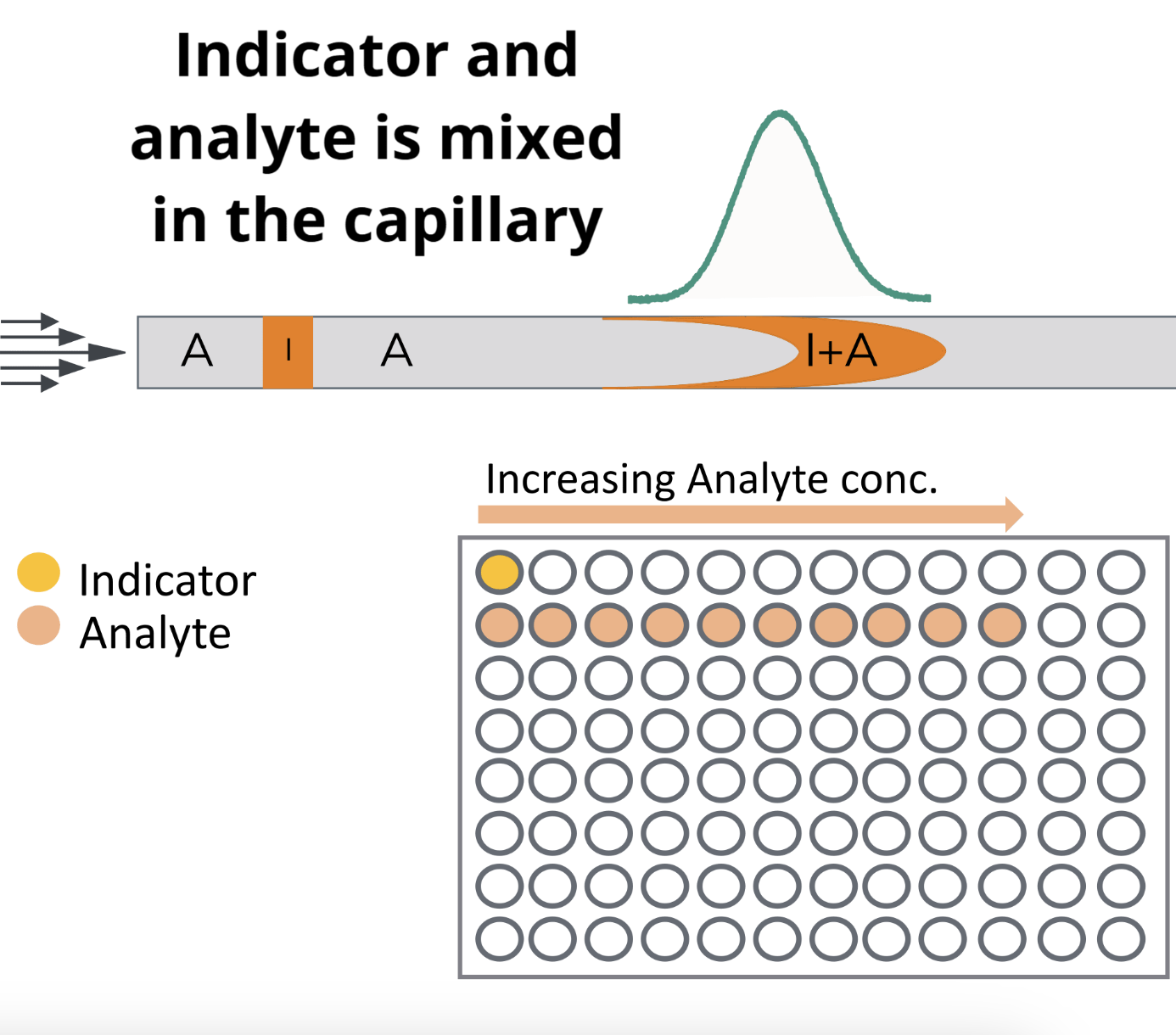
2. Capmix
Capillary Mix happens when the indicator and the analyte mix inside of the capillary. Here, there is only a single indicator position and a titration of analytes.
The capillary is filled with an analyte and the indicator is introduced with no analyte mixed in before the assay. When the indicator plug is mobilized with the analyte the indicator is diluted into the analyte.
Recommended sample requirements
- Minimum 10 µL indicator total
- 50 µL analyte per data point for a triplicate
- 100 µL of analyte stock at 20 fold the expected Kd
Recommended when assaying
- In solution kinetics (required)
- Assays where indicator is precious (e.g. membrane proteins)
- Assays relying on very small changes in Binding Related Intensity Change (BRIC)
Capillary dissociation
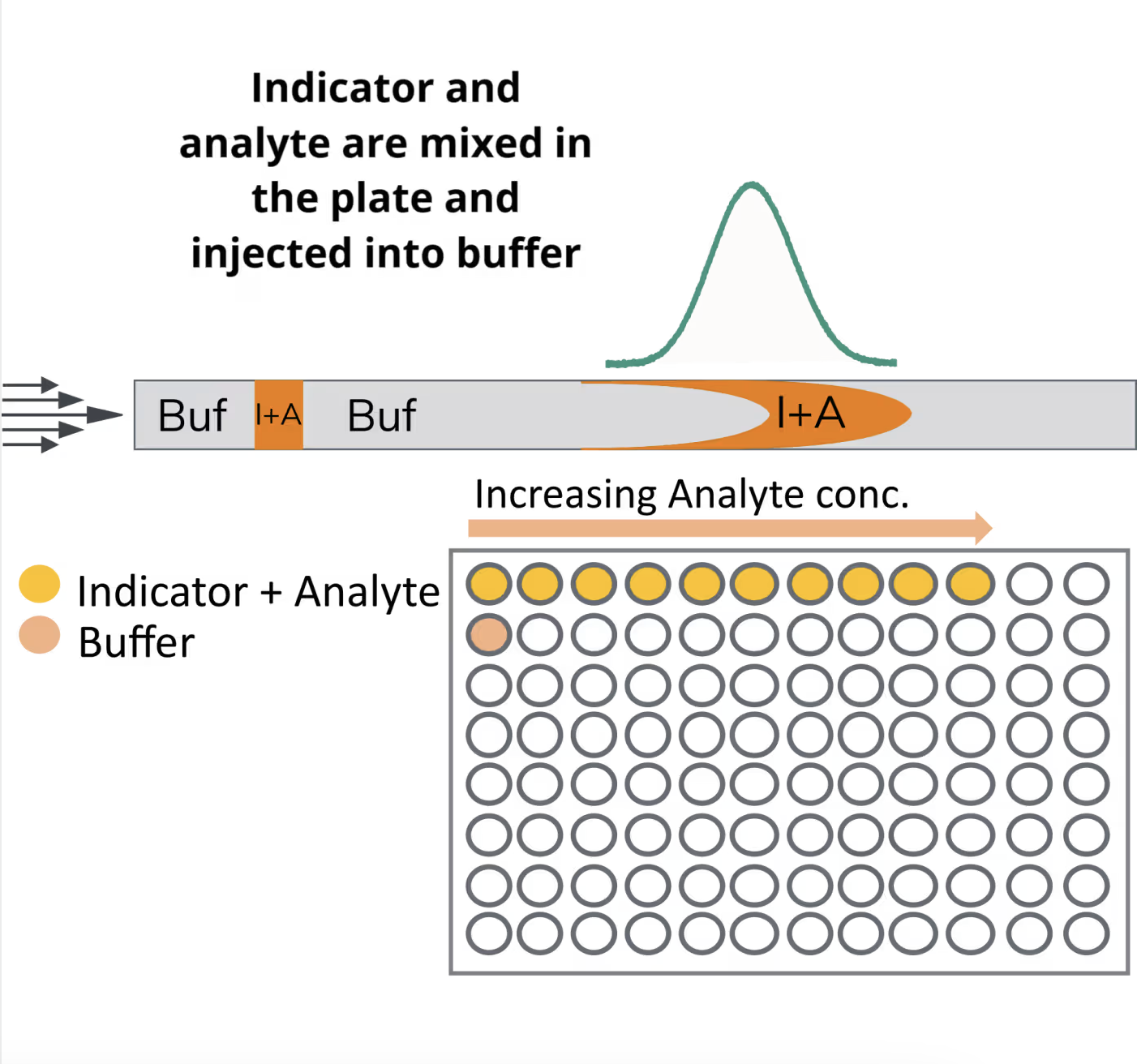
In a CapDis assay there is a series of indicators with increasing concentration of analyte. These are then injected into buffer. This causes a dilution of the sample potentially dissociating a given complex. In this type of assay the time to reach the detector is controlled by the mobilization pressure. Doubling the pressure will result in the sample arriving at the detector at half the time also cutting the potential dissociation time in half.
Sample requirements
- Minimum 10 µL indicator + analyte per data point
- A couple of µL buffer
- Analyte stock at 20 fold the expected Kd
Use this approach for/when:
- Large scale binding screen
- Indicator and analyte is extremely sticky
- Analyte is precious
Rapid Kd
Continuous Titration Based Spectral Related Intensity Change (cSpring) affectionately nicknamed "fast/rapid Kd".
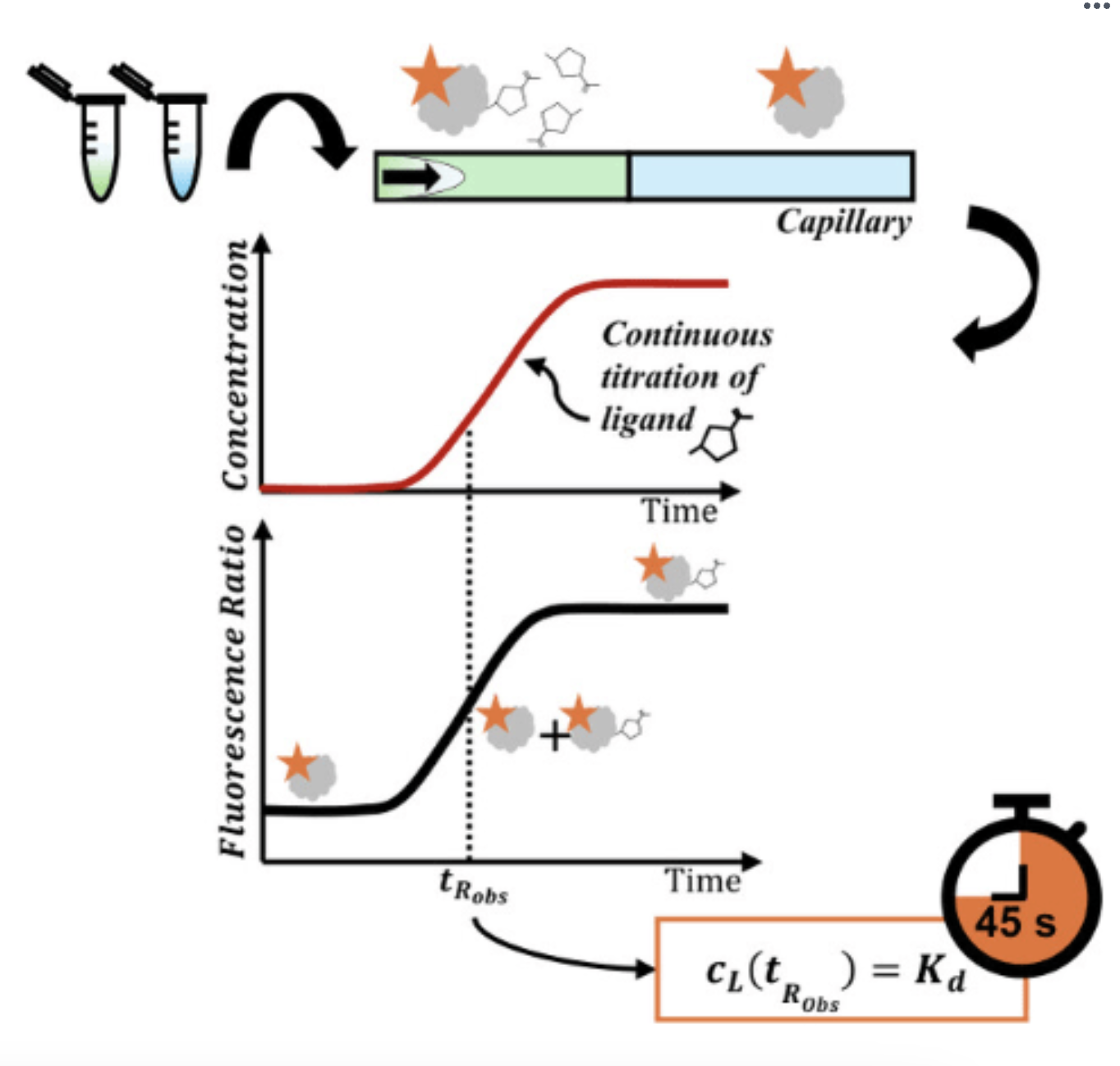
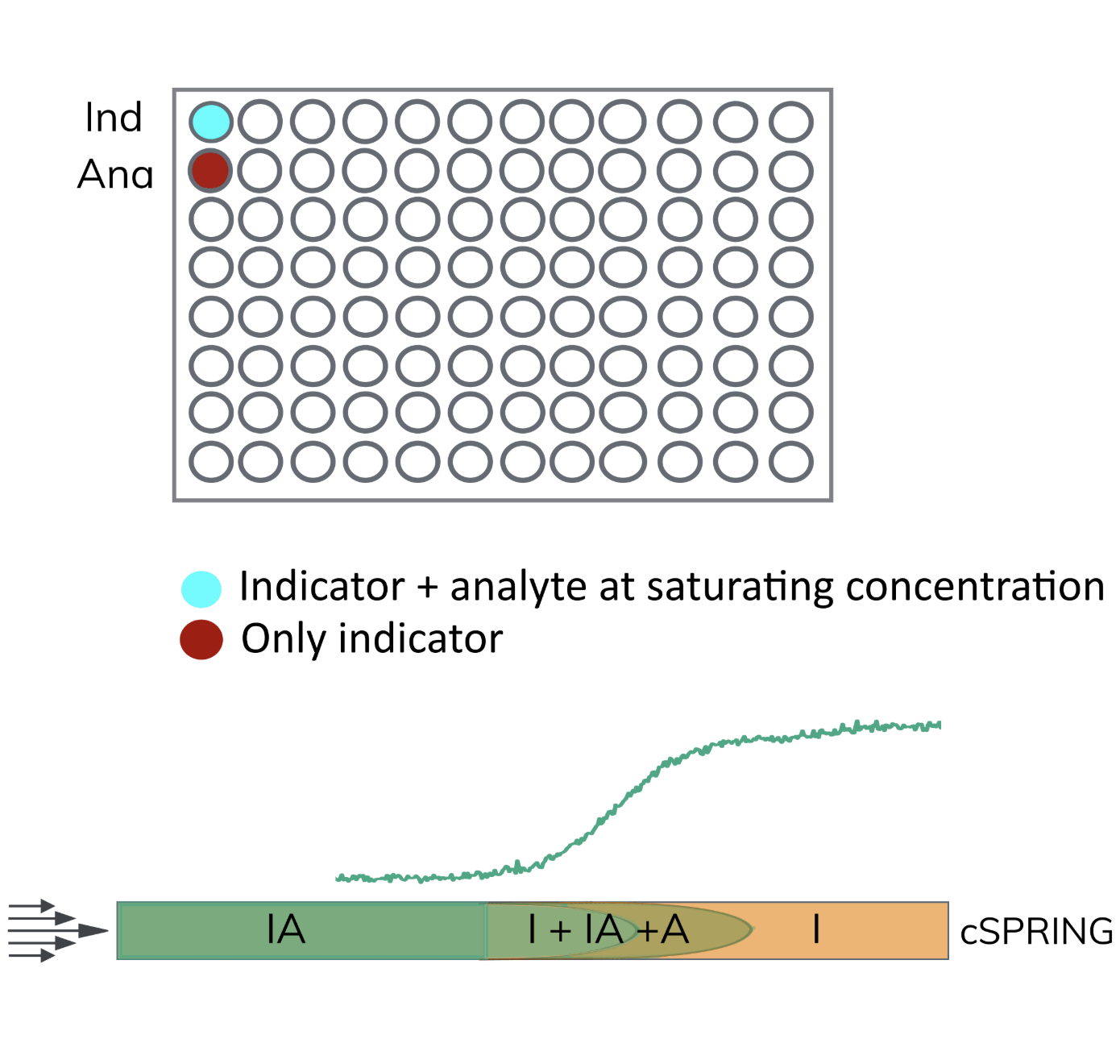
In a cSPRING experiment only a single indicator mixed with a saturating concentration of analyte and a single indicator without any analyte is required.
The capillary is filled with the free indicator (I) and the indicator mixed with the analyte is then mobilized though the capillary. This causes a concentration gradient of analyte to occur between the indicator phase and the indicator + analyte phase.
The indicator concentration remains constant. Learn more on our e-learning platform: https://signups.motimate.app/fidabio
Capflex & TDIPS
Capflex and TDIPS are more-advanced formats used in biomolecular research. You can learn more about this on our e-learning platform
